
Hi all,
In fact this is general chemistry question. But i think answers also can help food professionals.
Question is that;
When a solid is dissolved in a liquid, which size are the dissolved solids in solution? Nanometer, some of micrometer or both?
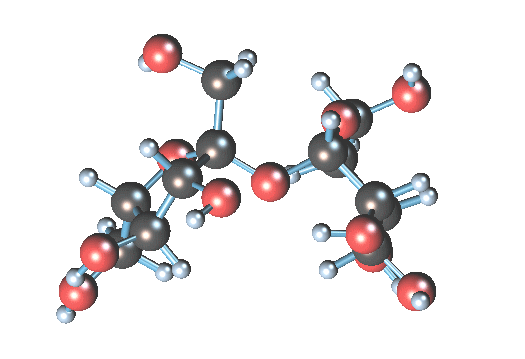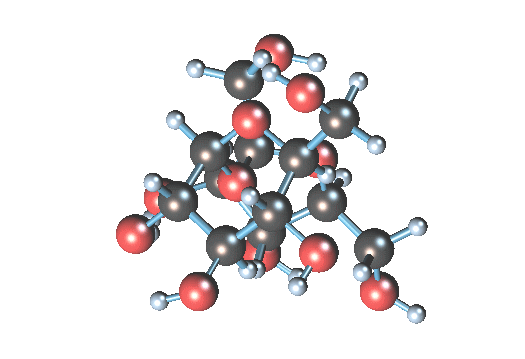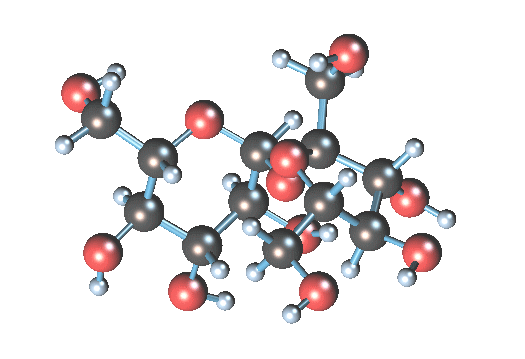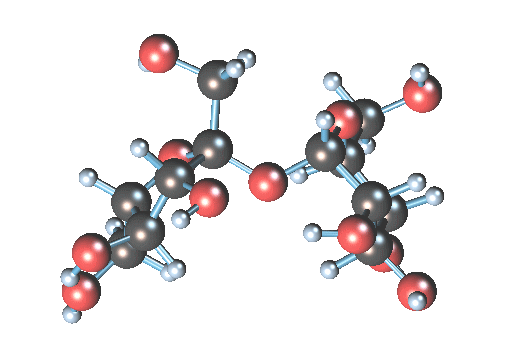
Sucrose molecule size is a = 1.08631 nm, b = 0.87044 nm, c = 0.77624 nm
so when sucrose is dissolved in water all molecules are alone or some of them can be together?
I think all molecules are not alone.
Sucrose gif is from Wikipedia
Molecule size info from Wikipedia
.